Week 3
ERROR ANALYSIS AND GRAVIMETRY ANALYSIS
Gravimetric Analysis & Precipitation Equilibria
by Tan Ching Mun (16002662)
In week 3, we were introduced to the topic of gravimetric analysis.
So, what is gravimetry analysis?
Gravimetry analysis is one of the tow analytical methods, namely gravimetric titrimetry, and atomic mass spectrometry.
As mentioned in the lecture notes, for gravimetric titrimetry which will be further discussed in this chapter, analyte concentration can be determined from the mass of reagent of known concentration required
to react completely with the analyte.
There are a few properties of precipitate in a successful gravimetric analysis as shown below
 The factors are:
The factors are:
In Precipitation Process, there are 7 steps involved, namely:
Step 1: Preparation of Analyte Solution
 Precipitation process is separated into 3 substeps.
Precipitation process is separated into 3 substeps.
So, what is gravimetry analysis?
Gravimetry analysis is one of the tow analytical methods, namely gravimetric titrimetry, and atomic mass spectrometry.

As mentioned in the lecture notes, for gravimetric titrimetry which will be further discussed in this chapter, analyte concentration can be determined from the mass of reagent of known concentration required
to react completely with the analyte.
There are a few properties of precipitate in a successful gravimetric analysis as shown below

- A gravimetric precipitating agent that reacts specifically or at least selectively with the analyte.
- Careful manipulation when forming and treating the precipitate.
In Precipitation Process, there are 7 steps involved, namely:
- Preparation of the solution
- Precipitation
- Digestion
- Filtration
- Washing
- Drying or Igniting
- Weighing
Step 1: Preparation of Analyte Solution

Step 2: Precipitation

- Supersaturation
- Nucleation
- Precipitation
Watch Me!

- The greater the supersaturation, the more rapid the crystal growth rate.
- Rapid growth rate may cause imperfections in the crystal & trapping of impurities.
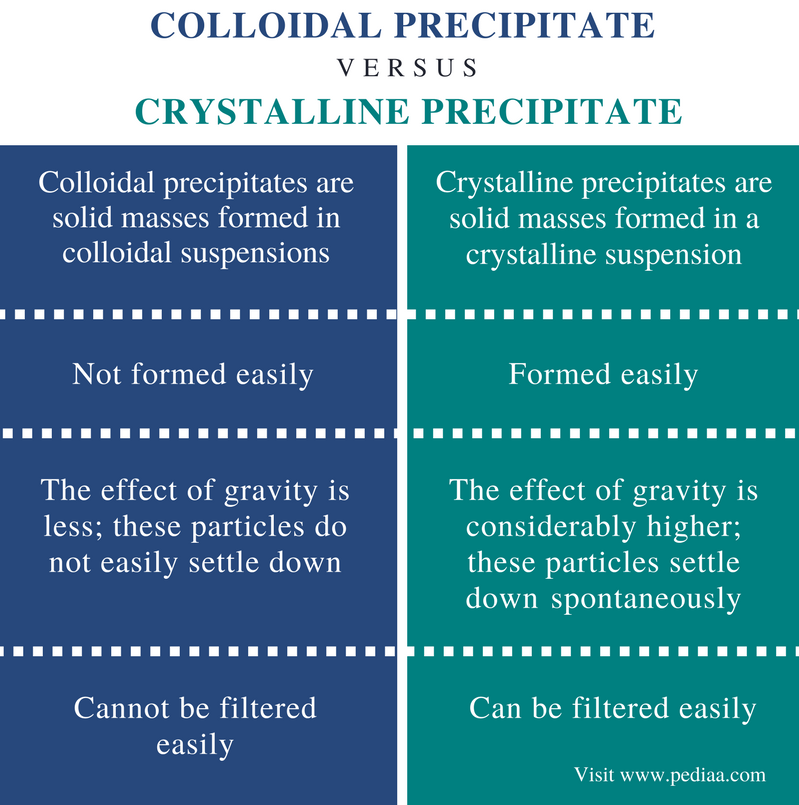
Crystalline Precipitate is preferable as it settles spontaneously & can be easily filtered.
Based on the von Weimarn Ratio, the particle size of precipitates are inversely proportional to the relative supersaturation of the solution.

To keep Q low and S high during precipitation, we could:
- Precipitate from dilute solution. (Q low)
- Add dilute precipitating reagents slowly, with effective stirring (Q low and prevent an excess of reagents)
- Precipitate from a hot solution. (High S)
- Precipitate at the lowest pH possible (High S)
- Add a little excess of the precipitating agent to check the completeness of the precipitation.
Step 3: Digestion (Ostwald Ripening)
Next, Ostwald Ripening is carried out to make larger and purer crystal.
Digestion is a process where precipitate is heated for an hour or more in the mother liquor to let larger crystals grow at the expense of smaller ones.
Digestion helps in:
- forming larger crystals
- reducing surface contamination
- reducing crystal imperfections
Next, I learned that Co-precipitation is the carrying down from the solution containing other constituents normally soluble causing the precipitate to become contaminated.
Here are some common types of co-precipitation:
Here are some common types of co-precipitation:

Precipitation from a homogeneous solution is more desirable as the precipitate formed is usually large and can be easily filtered.
This is because the precipitating agent is generated slowly in the solution of the analyte by a slow chemical reaction which immediately reacts with the analyte.
The relative supersaturation is kept low during the entire precipitation.

Step 4: Filtration
Colloidal crystals could be hard to filter as the particles are too small.
Filtration can be done in two ways:
i) Filtration through a filtering paper

Stirring rods are used as part of proper laboratory technique when decanting supernatants because the contact helps to negate the adhesion between the side of the glassware and the supernatant that is responsible for the liquid running down the side.
ii) Filtration through a filtering crucible (funnel)
ii) Filtration through a filtering crucible (funnel)

Step 5: Washing
Washing is important for removing mother liquor and co-precipitated compounds.
However, many precipitates cannot be washed with pure water as peptization will occur.
An electrolyte is used to wash the precipitate to avoid peptization.
The electrolyte chosen must:
Washing is important for removing mother liquor and co-precipitated compounds.
However, many precipitates cannot be washed with pure water as peptization will occur.
An electrolyte is used to wash the precipitate to avoid peptization.
The electrolyte chosen must:
- be volatile at the temperature of drying/ignition
- not dissolve the precipitate.
Step 6: Drying or Igniting
This step removes water and volatile electrolyte to produce dry precipitate.
Heating is usually at 110-120 °C for 1-2 hr.
Ignition (very high-temperature drying) converts precipitates to compounds more suitable for weighing.
Step 7: Weighing
Weighing of precipitate must be carried out carefully to reduce random errors, the analytical balance must be calibrated properly and static electricity must be avoided.
Gravimetry Calculations
This is the most important topic in this chapter.
Three Key Points
-
Chemistry
-
Stoichiometry
-
Composition Of Precipitate
Mol Ratio - Reaction Equation /Formulas of analyte and precipitate
Gravimetric Factor (GF) - the weight of analyte (substance sought) / unit weight of precipitate.

Below is the formula for calculating the % composition of the substance sought.

Sample Exercise


Type of Precipitating Agent
- Inorganic Precipitating Agents
Produces slightly soluble salts/ hydrous oxides - Reducing Agents
Convert an analyte to its elemental form for weighing - Organic Precipitating Agents
Forms slightly soluble non-ionic products & largely ionic products
Reflection
We had some revision regarding the previous chapter before the class starts. With the quiz question in mentimeter.com, we were able to understand the topic of discarding the questionable value using Q-test better. A clear explanation was given by the lecturer to help us recall and revise the topic.
This chapter is quite long and requires more time to understand thoroughly. However, I appreciate AP Dr Bawadi's effort to explain the points again when we were lost. I was not confident at first while calculating the gravimetric factor as I was confused about determining the analyte and precipitate, and also to balance the mole of the components. However, after going through the given exercise, I am able to do the exercises confidently.
We had some revision regarding the previous chapter before the class starts. With the quiz question in mentimeter.com, we were able to understand the topic of discarding the questionable value using Q-test better. A clear explanation was given by the lecturer to help us recall and revise the topic.
This chapter is quite long and requires more time to understand thoroughly. However, I appreciate AP Dr Bawadi's effort to explain the points again when we were lost. I was not confident at first while calculating the gravimetric factor as I was confused about determining the analyte and precipitate, and also to balance the mole of the components. However, after going through the given exercise, I am able to do the exercises confidently.
Tutorial 2
I have gone through the tutorial questions before the session was held. I don't understand some of the questions fully before the tutorial session starts. However, I am able to understand and redo the questions after the session.
Below are the question and provided answers during the tutorial session with some of my revision markups for better understanding. I hope it's helpful for you too!
1. Explain the difference between


2. What are the structural characteristics of a chelating agent?
A chelating agent is an organic compound that contains two or more electron-donor groups located in such a configuration that five- or six- membered rings are formed when the donor groups complex a cation.

3. Treatment of a 0.2500-g sample of impure potassium chloride with an excess of AgNO3 resulted in the formation of 0.2912 g of AgCl. Calculate the percentage of KCl in the sample.

4. What mass of AgI can be produced from a 0.512-g sample that assays 20.1% AlI3?

5. Ammoniacal nitrogen can be determined by treatment of the sample with chloroplatinic acid; the product is slightly soluble ammonium chloroplatinate:

The precipitate decomposes on ignition, yielding metallic platinum and gaseous products:
Calculate the percentage of ammonia in a sample if 0.2115 g gave rise to 0.4693 g of platinum.

6. A 0.2121-g sample of an organic compound was burned in a stream of oxygen, and the CO2 produced was collected in a solution of barium hydroxide. Calculate the percentage of carbon in the sample if 0.6006 g of BaCO3 was formed.

7. How can the relative supersaturation be varied during precipitate formation?

8. What mass of Cu (IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O?


9. Define
(a) digestion.
Process in which a precipitate is heated in the presence of the solution from which it was formed (mother liquor). Digestion improves the purity and filterability of the precipitate.
(b) adsorption.
Adhesion of atoms, ions or molecules from a gas, liquid or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by r permeates a liquid or solid, respectively.
(c) reprecipitation.
The filtered solid precipitate is re-dissolved and precipitated because the concentration of the impurity in the new solution is lower, the second precipitate contains less co-precipitated impurity.
(e) counter-ion layer.
Describes a layer of solution containing sufficient excess negative ions that surround a charged particle. This counter-ion layer balances the surface charge on the particle.
(g) supersaturation.
A solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances. It can also refer to a vapor of a compound that has a higher (partial) pressure than the vapor pressure of that compound.
10. The mercury in a 1.0451-g sample was precipitated with an excess of paraperiodic acid, H5IO6:
The precipitate was filtered, washed free of precipitating agent, dried, and weighed, and 0.5718 g was recovered. Calculate the percentage of Hg2Cl2 in the sample.


That's all from me for Chapter 3. See you in the next chapter.
